Currently, it is estimated that only one third of the population goes to a clinic to be tested and treated for malaria. The other two thirds of the population self-diagnose and treat the condition at home or don't treat it at all. Clandestine malaria treatments usually consist of paracetamol and less frequently anti-malarial drugs or natural medicines. There are certain dangers associated with this behavior. On one hand, self-medication has led to cases of toxicity due to overdosing. On the other hand, it enhances resistance of the malaria parasite when anti-malarial medication is used in insufficient doses. Under treatment occurs frequently due to the relatively high prices of anti-malarial medications. Some of the reasons for the low number of patients coming to the clinic include insufficient awareness of the risks of malaria (insufficient health education) and the high cost of treatment. For a large proportion of the population, anti-malarial drugs represent a substantial part of the yearly household income.
Musoma is located in the Mara region in the North West of Tanzania where a high risk for transmission is present all year round. This can be partly explained by the proximity of Lake Victoria. It is hard to predict how many malaria episodes the average adult experiences during a year, as symptoms may vary and can be confused with symptoms of other endemic diseases. Due to the intense drought over the past year (2005-2006), malnourishment is common and illnesses occur more frequently and more severely as a result.
The availability of an effective and affordable alternative for malaria prophylaxis would decrease the number of malaria episodes experienced by the local population and would improve health conditions in the region. Various malaria vaccines are currently being researched in clinical studies, however the clinical viability of such vaccines remains as yet unknown.4,5,6
Although Artemisinin derivatives have been proven effective in the treatment of malaria as single agents, single agent treatment is not advised. The suggested use is in combination therapy with anti-malarial drugs with a slow elimination rate. With this approach the eradication of the parasite is more complete and resistance will develop less rapidly.7
The list of described therapeutic applications for neem is extensive and the medicinal properties are widely implemented on the Indian subcontinent. The leaf and bark of the tree are commonly used in tea or in oily or aqueous extracts. Neem oil is produced by pressing the seeds or alternatively by soaking the leaves in vegetable oil. These preparations can be used for purposes as diverse as crop protection, insect repellent, treatment of various skin disorders as well as systemic bacterial, viral and fungal infections and for the prevention and treatment of malaria. Preclinical trials have shown that neem acts through two different pathways. One is by directly attacking the causative micro-organisms, the other is by boosting the host's immune system on both the humoral and the cellular level.8 According to our current knowledge, the use of neem in humans as a prophylaxis for malaria has not been described in published literature.
An additional characteristic of neem oil is that it produces durable, yet reversible birth control. A test was carried out with 20 male members of the Indian army for the duration of a year. Daily oral doses of neem seed oil were administered in gelatin capsules to twenty married soldiers. It took 6 weeks to reach 100% birth control and the effect remained during the entire year of the study. There were no new pregnancies during the trial. The infertility of the men was reversible within 6 weeks after cessation of the daily neem treatment. Preclinical research in male monkeys in India and the United States shows that neem reduces fertility without inhibiting libido or sperm production.8 Neem oil has further been used as an effective contraceptive in women when taken orally or applied intra-vaginally before sexual intercourse. It is also thought to have a preventive effect against sexually transmitted diseases when used topically before or after intercourse. This effect appears to be mediated by activating the local immune cell population in the vagina, thus increasing the antigen presenting ability which leads to a spermicidal effect.8
Numerous studies have been carried out to research the toxicity of neem leaf and bark when taken orally. It was determined in these studies that the neem leaf and bark are very safe to be taken orally, except when taken in large quantities. Neem oil has been researched thoroughly and regulatory agencies in several western countries have found it to be safe when taken in low dosages for a limited amount of time. Some people taking neem seed oil internally experienced abdominal discomfort and nausea. Also consumption of large amounts of raw neem oil has been implicated with decreased liver function. It is yet unclear whether these side effects can be explained by impurities introduced to the oil by low quality manufacturing processes. Further research with pure forms of the seed oil is needed to provide clarity on this matter As neem remains a natural compound with various active ingredients, it is always advised to remain vigilant in its use. As applicable to any medicinal compound, overdosing can potentially be harmful. Such effects are not expected with the homeopathic neem preparation for two reasons. Firstly, the solution is prepared with the neem leaf which has been found safe when used in reasonable quantities. Secondly, the concentrations of the neem leaf are extremely low in homeopathic preparations. Homeopathic medicines are rarely implicated with side-effects.
The Abha Light clinics in Kenya have been using neem in its various forms for the treatment of many different illnesses as it is cheap and easy to get in areas where modern medicines are scarce. The Abha Light Foundation in Nairobi and its 8 affiliated clinics have been treating many patients with homeopathic neem drops since 2000. Amongst others, the clinic has used the homeopathic medicine for the prevention and treatment of malaria. To date, the clinic has had very favourable experiences with this treatment. The cost of production for the homeopathic neem medicine is extremely low, averaging around a few cents USD per 20 ml. bottle which will last one patient up to 2-3 months when the drops are taken prophylactically. Interestingly, the bottle is the most expensive part of the treatment, making up about 60% of the total cost. The cost of one bottle of neem tincture remains well under the price of 0.5 US dollars.
The beneficial properties of neem and the lack of side-effects in this preparation combined with the low manufacturing cost may constitute a locally viable alternative to the more expensive anti-malaria medication. If proven effective as a prophylactic it may provide a much needed answer to the current upsurge in malaria cases in developing countries.
For this purpose an exploratory trial was set up to research the effects of the homeopathic neem remedy in subjects from the town of Musoma and surrounding villages in Tanzania. This was a single arm, prospective, observational trial in subjects with recurrent malaria attacks. The study started in June 2005 and has been ongoing for a little over 6 months at the time of writing. Both children and adults were included in the trial. The trial population was a group of orphans supported by the Tanzanian NGO Foundation HELP and their guardian families. Pregnant women were excluded from the study due to the experimental status of the medication.
The choice was made to observe the effects of the study remedy in an at-home setting. From the start, it was anticipated that this set up would pose some challenges in acquiring reliable data. Obtaining information about treatment compliance especially would require a specific effort as subjects could not be monitored regularly. A clinical setting was not feasible due to the daily regimen and the relatively long follow up. The benefit of a trial in an at-home setting is that it reflects accurately how the remedy is used by the subjects and which efficacy is related to this use. The use of diaries was not feasible due to the widespread existence of illiteracy in certain parts of the population. As this was an exploratory trial, the efficacy data was based on subject reports. Malaria symptoms were not objectified by a clinical diagnosis. To maximize the consistency of the results, all subjects were visited in their homes by the same local social worker. This person was well known by the guardian families included in the trial and he provided the instructions on the correct use of the remedy. In addition, the medication was dispensed by the same person. All subjects were interviewed using an identical questionnaire. This questionnaire was always completed by the same social worker. In most cases, the primary guardians reported on the experiences with the remedy for the entire family.
All subjects were requested to take the neem medication as follows: children - 3 drops per dose twice daily in a glass of water, adults - 5 drops per dose to be taken either directly into the mouth or in a glass of water. In the event of a malaria attack, the adults were instructed to take 15 drops every hour until the symptoms subsided. The instructions for the children were to give them 10 drops every hour identical to the adults.
The subjects were advised to have a week drug holiday after finishing every bottle to prevent any potential side-effects which have previously been associated with the continuous use of other neem preparations. To reinforce the drug holiday, the subjects had to request a renewal of the bottle after finishing the previous bottle. Only after request, subjects were issued a new bottle. Any pregnancies during the study treatment were to be reported immediately.
The patients were visited before the start of the trial and at 3 months and 6 months after the start of preventative treatment to assess compliance with the study drug and the efficacy of the treatment regimen. Baseline characteristics comprised of age, sex and a positive number of malaria attacks in the previous 12 months in a yes/no answer format. The instructions for the correct use of the medication and the dosage were repeated in every household at 3 months after the start of treatment. Any change in the frequency of malaria attacks were recorded after 3 months and 6 months of preventative treatment, again by the same social worker. Subjects were asked to indicate whether the frequency of malaria attacks had decreased, remained the same or increased after the start of the study treatment. Compliance information was obtained as well as the occurrence of any pregnancies during treatment when applicable. The study is currently ongoing, final results will be presented after a two year follow-up.
Considering the exploratory nature of the study, no statistical significance testing was planned. The results were reported using descriptive statistics. In order to detect a decrease in the proportion of subjects experiencing malaria attacks from 80% to 70% over 6 months, with a two-sided alpha of 0.05 and power of 80%, a sample of size 136 subjects would be needed. Clearly, this study with a sample of size 152 subjects has a sample size large enough to detect a 10% decrease in proportion of malaria attacks.
This publication presents the 6 months follow up data obtained from June until December 2005.
A total of 152 patients were enrolled in the trial. The treatment group consisted of 79 children up to and including the age of 18 years and 73 adults above the age of 18 (mostly guardians of the researched children). The group of children consisted of 37 girls and 42 boys. The mean age of the children was 11 years (range 4-18). The group of adults consisted of 48 women and 25 men. The mean age of the adults was 38.6 years (range 19-93). All subjects reported at least one malaria episode in the previous 12 months.
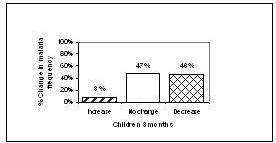
At the 3 month check up, 8% (n=6) of the children had an increase of malaria episodes compared to baseline, in 47% (n=37) of the children no change was observed in the frequency of episodes and in 46% (n=36) a decrease of malaria episodes was reported. In the adult group, 1 subject reported an increase in malaria attacks, 29% (n=21) reported no change and 70% (n=51) reported a decrease in the frequency of malaria episodes.
After 6 months, 10% (n=8) of the children had an increase of malaria episodes, in 16% (n=13) no change in frequency was observed and in 73% (n=58) the number of malaria attacks had decreased. With the adults, 3% (n=2) reported an increase of malaria attacks, 8%(n=6) reported no change and 89% (n=65) reported a decrease in malaria attacks.
In the study population as a whole, 5% reported an increase of malaria attacks at 3 months and 7% at 6 months. 38% reported no change at 3 months decreasing to 13% at 6 months. The percentage of subjects who reported a decrease of malaria episodes increased from 57% at 3 months to 81% after 6 months of treatment. See Table 1 and Figures 1-4.
Up to 68% of the children took less than the prescribed amount of study drug in the first 3 months. In contrast, 89% of the adults took the medicine as intended or took more than the intended prophylactic dose.
38 of the women in the adult group were within the fertile age during the trial and 20 girls in the children group may have been fertile, when a cut-off point of 12 years is applied. In response to the pregnancy question, no women indicated having been pregnant during 6 months previous to the start of treatment and there were no reports of pregnancies during the trial. There was thus no difference in frequency of pregnancies before and during the trial.
| 3 months | 6 months | ||||||
| No. | Higher | No change | Lower | Higher | No change | Lower | |
| Children (<=18 years) | 79 | 6 (8%) | 37 (47%) | 36 (46%) | 8 (10%) | 13 (16%) | 58 (73%) |
| Adults (>18 years) | 73 | 1 (1%) | 21 (29%) | 51 (70%) | 2 (3%) | 6 (8%) | 65 (89%) |
| Total | 152 | 7 (5%) | 58 (38%) | 87 (57%) | 10 (7%) | 19 (13%) | 123 (81%) |

Figure 1. Change in malaria frequency from baseline at 3 months (children = 18 years)
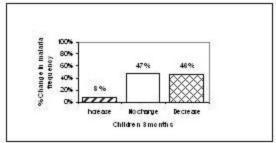
Figure 2. Change in malaria frequency from baseline at 6 months (children = 18 years)
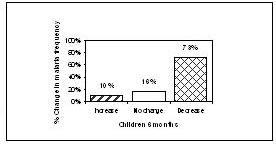
Figure 3. Change in malaria frequency from baseline at 3 months (adults> 18 years)
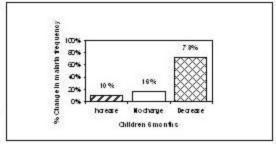
Figure 4. Change in malaria frequency from baseline at 6 months (adults> 18 years)
Despite active prompting for side-effects at every visit, no side-effects were reported during the study treatment.
This study was a prospective, exploratory trial to research whether daily use of homeopathic neem medicine could be administered safely and effectively for the prevention of malaria attacks in subjects with a high risk of contracting the illness. The study was carried out in the Mara region of Tanzania, which has a continuous high risk of transmission of plasmodium falciparum infections all year round. Children and pregnant women are particularly vulnerable to malaria due to insufficient intrinsic immunity against the disease. Pregnant women were excluded from the current trial, due to the experimental nature of the study drug and unknown side-effects. About half of the study population consisted of children of various ages, ranging from 4 to 18 years. There was a homogenous spread of all ages within this group. Both girls and boys were researched in an almost 1:1 ratio, making this a balanced group. The ages of the participating adults also showed a homogenous spread between the ages of 19 and 70 with the exception of one woman who was 93 years old. There was a slight predominance of women in this group, probably due to their role as caregivers in the family and their usually higher interest in health matters compared to men in this region. The study population contained more children than the average local population. As children are more susceptible to malaria, it was deliberately planned to acquire more data from this group. The planned sample size was met and was large enough to allow for reliable conclusions in this non-comparative trial.
From the efficacy results, it is clear that the subjects predominantly reported a decrease in malaria attacks. As the diagnosis of malaria was not clinically confirmed, a placebo effect may have biased the results towards the positive. Also, a recall bias cannot be ruled out. Furthermore, self-diagnosis can be tricky as symptoms of malaria can mimic other endemic diseases. The effect on the results of this bias is difficult to quantify. Having said that, the continuing decrease in reported attacks between 3 and 6 months of treatment in both groups, seem to indicate that there is indeed a treatment effect. In this case, as the treatment duration increases, the effect becomes more evident as the number of experienced malaria attacks on study medication deviates further from the baseline frequency. It would be interesting to see if this decline is sustainable during the remaining 18 months of the trial.
The results indicate that the preventative treatment effect in this study was higher in the adult population than in the group of children. In our opinion two reasons could explain this difference, as discussed in the following section.
As already mentioned, children constitute one of the two vulnerable groups in the epidemics of malaria. The relative incidence of malaria is larger in this group and the course of the disease is more often serious than in adults who have had a chance to build up a certain immunity. This would explain both the lower overall response in the children after 6 months and the slightly higher percentage of children with an increase in malaria attacks while on treatment. We searched our dataset for common factors in those subjects where the malaria frequency had increased both after 3 and 6 months. No common factors could be identified between the individual cases. Only two of these 8 cases were known to be HIV positive. Drug intake was not significantly different than the rest of the treatment group and the effect could not be attributed to other concurrent illnesses or additional factors. We therefore conclude that the higher proportion of malaria increases must be due to the higher baseline susceptibility of children to the disease.
Additionally, the results show a difference in timing of medicine effect between the adult group and the children. In the adult group the largest decrease in malaria attacks occurs between the start of treatment and the first visit after 3 months. This observation is in line with previous experiences in other clinics. In contrast, the main decrease in the group of children occurred between the second and the third visit. This late decline can be explained by a below average drug intake during the first three months of treatment. Neem drops are very bitter to the taste, and are therefore difficult to take orally, especially for young children. Despite the instructions to add the drops to a glass of water for the children, it was only after reiteration at the 3 month follow-up visit that this practice was implemented. With the drops being added to water, drug compliance increased leading to a larger proportion of decreases in malaria episodes in the second half of the trial. This experience portrays one of the challenges of clinical research in an at-home setting in a developing nation. Understanding and execution of verbal instructions is a limiting factor when part of the study population has little to no formal education. The reliability of such data could be greatly improved by the use of subject diaries. This would however restrict the research setting to a more developed country, where illiteracy is less common. Malaria is however generally not endemic in those areas as education and literacy in a large percentage of the population require a certain degree of development of a nation.
As previously described, the secondary objective for this trial was to research whether there was evidence of a birth control effect of neem when used in the homeopathic preparation. Approximately 58 girls and women could have been expected to be in the fertile age during the trial. The questionnaire did unfortunately not include a question on the existence and frequency of sexual activity among the subjects during the treatment, hereby making it impossible to conclude whether the study medicine had a birth control effect. The absence of a difference between the number of pregnancies before and during the trial is therefore not conclusive. More attention should be given to this aspect during the set up of a future trial.
The treatment effect was directly linked with compliance to the treatment regimen, as observed in the group of children where the number of reported malaria attacks clearly decreased after the neem drops were added to drinking water. Our experience in this trial is that it is imperative to add the neem drops to a glass of water to ensure compliance in small children as the remedy is very bitter to the taste. Also for adults, it is advisable to add the drops to drinking water.
The lack of side effects found in this trial concurs with the previously reported safety of homeopathic medicines. In this study there was no evidence of short term or acute side-effects after 6 months of preventative treatment. The follow up period of this trial was not long enough to explore long-term side effects. Specific clinical trials should be designed to gather this type of information. As no laboratory investigations were performed during this trial, there is no information on potential liver enzyme elevations. There was no clinical evidence to suggest impairment of the liver function in the researched subject. One would not expect such effect with a homeopathic preparation, as homeopathic preparations only contain trace amounts of the original medicinal compounds. Also, the decrease in liver function was only associated with the use of crude neem oil. This oil is derived from neem seeds and not from neem leaves as utilized for the preparation of the study medication.
With every newly developed anti-malarial medicine there is the perpetual question of resistance. With most modern medicines, resistance of the malaria parasites is found relatively soon after introduction. Modern medicines generally contain only one purified active substance of the original plant to which substances are added in the formulation process. It is thought that pathogens may develop resistance more rapidly to the single active substance. In natural remedies, the natural balance of all substances is retained as found in the original plant. As the National Research Council (NRC) points out, Neem has a complex chemical makeup with more than twenty compounds identified to date. This theoretically makes development of resistance unlikely.9 It is unknown whether the homeopathic neem remedy produces resistance of the malaria parasite. The dual action of neem on both the parasite and the host immune system, may theoretically delay a decrease in clinical efficacy due to resistance. Furthermore, homeopathic remedies have not shown to produce resistance in the past, making the researched medication an interesting candidate for future trials. Obviously, this issue should be closely monitored as the use of neem medications expands.
From this trial we conclude that the researched homeopathic neem drops are effective and can be safely used up to 6 months when a drug holiday of a week is observed after every 2-3 months of treatment.
The results from this study show that the daily use of neem in a homeopathic solution is associated with a convincing reduction of malaria attacks in a large proportion of the study population. This effect was observed in both adults and children after 6 months of preventative treatment. More research is needed to clarify the durability of this preventative effect in the long term. Due to insufficient information the trial remained inconclusive towards the birth control effect as associated with neem. This aspect should be the subject of future trials.
The homeopathic neem preparation has shown to be effective for the reduction of malaria attacks in a highly endemic area for plasmodium falciparum. The treatment is safe in the short term and the low cost of manufacturing renders this treatment especially attractive for developing countries as the purchase cost is well within the range of an average household budget.
In response to the experiences in the above trial, our group is currently setting up a second trial in the same region of Tanzania incorporating blood parasite counts and including additional questions on the sexual behavior of the trial subjects to objectify the effects of homeopathic neem drops in an at home setting.
This trial was organized and financed by Global Resource Alliance (GRA), a non-profit organization in Ojai, California, USA.
Data was collected by Christopher Gamba, a social worker at Foundation HELP in Musoma, Tanzania.
We thank Didi Ananda Ruchira, Director of Abha Light Foundation in Nairobi, Kenya for her contributions to the body text and for her generous help and advice involving the study medication.
We thank Mr. Anselm Magoma, public health officer for the Mara region of Tanzania, for reviewing the article and for providing the necessary national epidemiological statistics.
We thank Mrs. Melanie Poulin-Costello, Statistician at Bayer Corp. in Toronto, Canada for her advice in the statistical section.
We thank Dr. Makuke for his cooperation and research into the use of neem tincture for the treatment and prevention of malaria.
A Voluntary worker for GRA (Global Resource Alliance), Musoma,
Tanzania
B Social worker in employment of Foundation HELP, Musoma,
Tanzania
C Director of Global Resource Alliance (GRA) in Ojai,
California, USA.
Brought to you by: ProductosdeNeem.com